Over 200 fellow healthcare leaders joined the 17th Annual Healthcare Finance and Growth conference for interactive programming discussing the latest developments in the industry and the challenges the industry faces in 2024 and beyond. With substantive panels and ample networking breaks, this year’s conference provided opportunities to connect with and learn from industry colleagues on how best to navigate opportunities, emerging subsectors and unique challenges of the healthcare industry.
Across 15 panels and 57 speakers, the conference provided deep dives on a number of topics and trends, encompassing numerous and varied insights from our participants. Key takeaways from the conference are below.
Consumer-Driven Healthcare Developments
There continues to be a rise in social media’s influence on healthcare. Healthcare consumers continue to evolve and are searching for information about their care and providers online. Significant evolution of the healthcare consumer can be attributed to the growing influence of social media on patient choices. Platforms like TikTok and Facebook have given users unprecedented access to information about health, wellness and treatment options. In the past, healthcare consumers relied primarily on word of mouth, TV advertisements or doctor recommendations when making decisions about treatments, products or services. Now, they increasingly turn to social media to influence these choices or validate information from providers. Before booking an appointment or trying a new treatment, many consumers look for reviews, testimonials and user-generated content. TikTok, for example, offers real-time feedback from users who document their health journeys. For a consumer, the allure of these platforms lies in their ability to present complex healthcare topics in bite-sized, easily digestible formats. Recognizing the impact of social media, healthcare companies have adjusted their marketing strategies accordingly. Previously, traditional advertising methods like print, television and radio dominated the healthcare industry. However, companies have increasingly begun to allocate a significant portion of their marketing budgets to social media. Healthcare companies must navigate this landscape carefully, balancing engagement with consumers while ensuring that accurate and reliable information is at the forefront of their content.
Telehealth continues to expand access to providers while simultaneously intensifying competition. Telehealth has rapidly evolved into a critical component of medicine. One of the most significant benefits of telehealth is its ability to extend healthcare services to broader, and sometimes underserved, populations. Telehealth bridges this gap by allowing patients to receive care without traveling long distances or enduring long wait times to see a specialist. While telehealth offers clear benefits in terms of access and convenience, it has also introduced a new level of competition within the healthcare industry. The growth of telehealth has also attracted a wide range of new players to the healthcare industry, including tech companies, startups and retail giants. Companies like Amazon, Google and CVS have ventured into telehealth, leveraging their technological expertise and vast resources. As more providers, health systems and even tech companies enter the telehealth space, the landscape is becoming increasingly competitive.
Deep Dive Into the Antitrust Climate
With federal and state antitrust scrutiny ever increasing in the healthcare space, healthcare entities should create a culture in which antitrust considerations are integral to transactional and ongoing operational decision-making. From the growing prevalence of state-specific, material transaction notice/consent laws to the expanding limitations placed on non-compete restrictions, antitrust scrutiny has increased significantly in recent years, especially in the healthcare industry. Such regulatory review has also deepened over time, with industry insiders noting that regulators are beginning to analyze competitive impacts at a specialty, sub-specialty or even specific provider level in situations where, historically, regulators would have only considered the impact, at a high level, to availability of care post-transaction.
In response, it is important for healthcare entities to proactively consider antitrust effects to avoid creating unnecessary risks or exacerbating those that already exist. For example, in connection with operations, healthcare entities should ensure that processes are in place to accurately and clearly track data that can support claims of improved patient and provider outcomes. In the deal context, healthcare entities should engage antitrust advisors early in the exploratory phase to help identify market opportunities and risks related to potential targets, establish strong gating diligence questions to flag possible issues (such as those related to payor contracts) and strategically craft risk-shifting provisions in the transaction documents in response. It will be far easier and less costly to avoid or address issues from the very beginning of a deal than to do so when the deal is already underway, close to completion or—worse—already closed.
Life Sciences Regulatory Essentials: What Investors Should Know
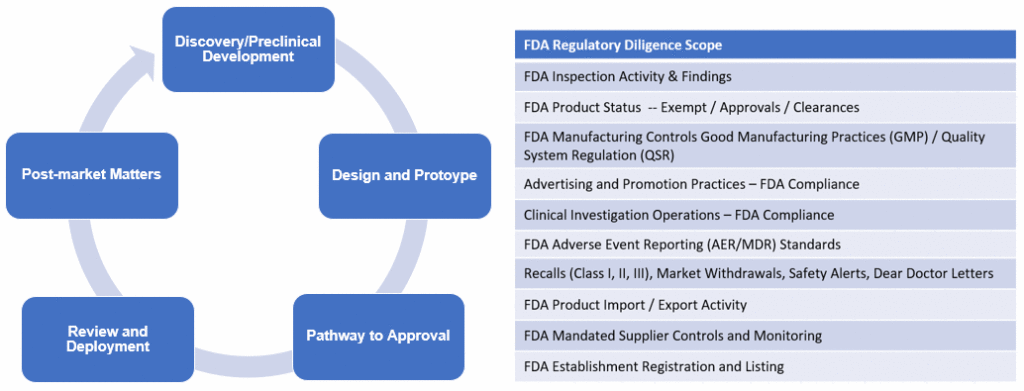
Investors are increasingly drawn to the life sciences sector due to its potential for high returns, which are being driven by groundbreaking innovations in biotechnology, pharmaceuticals and medical devices. However, investing in this sector also presents unique challenges, particularly around the regulatory framework that governs the development and delivery of life science products and services. The life sciences sector operates in one of the most heavily regulated environments, facing oversight from multiple state and federal agencies and global health authorities. Accordingly, regulatory compliance is a critical aspect of operating in this sector. Attention must be paid to ensuring compliance with the applicable regulatory regime throughout a product’s lifecycle, including with respect to clinical trials, product approvals, manufacturing, marketing and promotion, and post-market surveillance in the life sciences sector.
In furtherance of ensuring compliance, investors should conduct thorough due diligence to assess whether the target company is compliant with critical requirements imposed by the Federal Food, Drug, and Cosmetic Act. For example, with respect to clinical research sites, site management organizations (SMOs), and contract research organizations, due diligence should evaluate good clinical practice compliance, prior FDA inspections and observations, and sponsor relationships and clinical trial agreements, among other things. In the context of pharmaceutical manufacturing and distribution, diligence should evaluate a target company’s compliance with good manufacturing practices requirements to ensure that the target company’s manufacturing processes and facilities meet regulatory standards. In the medical device space, investors must pay close attention to a target’s compliance with the quality system regulation, marketing clearances and approvals, and filing of adverse events (known as medical device reports) with the FDA. Additionally, investors should carefully scrutinize a target company’s history of regulatory compliance more broadly, as any ongoing or potential litigation, government investigations or other regulatory action can lead to significant financial liability, reputational damage and operational disruption.
Life Sciences Regulatory Essentials: Due Diligence
The Expansion of Pharma Services and Life Sciences Investments
Interest in pharma services investment continued to grow throughout 2024, and industry insiders expect that this trend will continue as investment interest shifts away from traditional health care provider platforms. As the U.S. Food and Drug Administration encourages decentralization of certain clinical trial elements, more healthcare providers are eager to offer clinical trial options to their patients as part of a shifting standard of care, particularly in rural and underserved communities. This expansion has propelled an increase in entities providing clinical trial support services, such as clinical research organizations, SMOs and development of clinical research networks. While many CROs and SMOs historically specialized in niche clinical-trial support, such as oncology, rare blood diseases and pediatric indications, panelists indicated that they are seeing a shift toward consolidation in CROs and SMOs this year, and they expect that consolidation to continue into 2025. Further, those entities in the pre-clinical trial space — i.e., those conducting and supporting animal or in vitro studies — have begun exploring upstream expansion into support services for human trials.
On the commercial pharma services front, investment in 503A and 503B pharmacies, particularly on the heels of the GLP-1 boom, continues to expand. However, questions remain as to whether this boom reflects the “new normal,” or whether growth in such pharmacies will slow after shortages of GLP-1s resolve and the drugs are again commercially available. Further, interest in specialty drug manufacturing, bulk manufacturing and fill-finish operations remains strong, though consolidation is likely to continue as costs to manufacture continue to climb.
Looking ahead to the future, the panelists expressed optimism for continued interest and growth across pharma services for the remainder of 2024 into 2025, particularly in light of the Federal Reserve’s recent interest rate reduction.
The Intersection of Health Systems, Independent Providers and Healthcare Investors
Despite historic, financial, and cultural challenges, attractive alignment opportunities exist among health systems, independent providers and healthcare investors. After decades of separation and competition between health systems and independent providers, industry insiders are seeing both sides begin to break down historical barriers and embrace highly attractive alignment opportunities that can help them collectively improve patient outcomes, efficiency, scale and access — each of which are critical in an era of increasing costs, decreasing reimbursements and an increasing focus on patient outcomes. Alignment opportunities can range from co-management arrangements and recruitment strategies to joint expansion into new markets. For example, some systems are starting to move further into the management space and even partner with private equity to create new models of engagement that allow physicians to retain their independence while still seeing the benefits of a community-based model. Before embarking on one of these opportunities, however, industry insiders emphasize the importance of investing time and money on the front end to truly understand their own strengths and weaknesses, what they are trying to accomplish through a partnership, and which potential partners exist. The right partner must fit legally, operationally, financially, and, often most challenging, culturally to avoid a rocky relationship and a potentially costly and complex unwind down the road. The key is ensuring that partners align on overarching goals, establish clear data-driven processes and measures of performance, meaningfully share economic value in a legally compliant way, and provide for diverse stakeholder representation (such as ensuring that the physician voice is formally represented). While often challenging, these alignment opportunities can provide significant value to systems, providers, investors and patients alike when done properly.
Enforcement Considerations for Private Equity Investments in Healthcare
Recent trends in False Claims Act enforcement have encouraged healthcare providers to self-disclose their improper billing practices at a higher frequency than in years past. McGuireWoods LLP partners discussed how healthcare providers looking to correct improper billing practices (e.g., improper use of modifiers, misunderstanding E/M coding levels) have two primary choices: refund the money resulting from improper billing practices or self-disclose their conduct to the government. Historically, under the Office of Inspector General’s self-disclosure mechanism, the government would require payment of the amount of money improperly billed and collected plus 50% or more. Recently, this “plus” amount required by the government has decreased, in some cases down to the amount of money improperly billed and collected plus 10%. While this is still more than simply refunding the improper collections, there is a critical distinction: That extra amount paid provides greater protection from liability to the government, since a refund offers no formal release with respect to the conduct while a self-disclosure will lead to a formal settlement. Before refunding any improperly collected money to the government, therefore, a healthcare entity should speak with an experienced healthcare litigator about the possibility of paying a premium (smaller than past year) in exchange for a formal settlement and potential release with respect to the disclosed improper billing.
Clearwater on PE Hub: How Cyber Risk is Changing Private Equity
Industry participants put increased focus and attention on cybersecurity. During the “Clearwater on PE Hub: How Cyber Risk is Changing Private Equity” breakout session, panelists outlined that healthcare investors’ cybersecurity efforts are facing increased scrutiny by internal and external stakeholders as well as federal and state regulators. In addition to several high-profile attacks that drew national attention, 2023 saw increases in the total volume of ePHI breaches and ransomware attacks in the healthcare industry as well as increases in the total average dollar amount of revenue loss associated with a breach per healthcare entity. Furthermore, the healthcare industry, for the 13th consecutive year, had the highest total dollar amount of loss associated with breaches and attacks of any industry (when compared to all other industries in the United States). Factors contributing to such increases include, but are not limited to, the growth of attack surfaces via the digital space, the increase in the number of attackers orchestrating such attacks, and the increased likelihood that healthcare entities will pay ransom requests. Panelists suggested that healthcare investors, regardless of size, should take time to thoroughly review and ensure that robust compliance programs are in place for both currently owned entities and potential transaction targets. As one panelist noted, “It’s not a matter of if a breach or attack will happen, but when the breach or attack will happen.” Investors should create, modify, and/or maintain compliance programs to have clear and specific policies and procedures in place to address HIPAA and data privacy issues as they arise as well as regularly conduct proper evaluation and assessments of an entity’s cybersecurity capabilities. This likely implies the need for third party reviews and audits. Such actions can help an investor be adequately prepared to take immediate action when a breach or attack occurs.
Innovation in Healthcare Delivery and Technology
Value-based care continues to evolve to better align patients and providers with CMS’s goals for care relationships. The pandemic acted as a catalyst, driven by necessity, for the rapid adoption of virtual and innovative health platforms. These developments have enhanced care delivery and have set the foundation for achieving the Centers for Medicare & Medicaid Services’ (CMS) goal of ensuring Medicare beneficiaries and most Medicaid beneficiaries establish longitudinal, accountable care relationships by 2030. However, providers and physicians continue to face headwinds in risk-based and value-based care models, as hesitation and fatigue arise surrounding the adoption and delivery of these new services and technologies. Patients also encounter barriers due to high acuity and accessible care. Accounting for these challenges, regulatory changes are trying to realign incentives for providers to improve outcomes at lower cost. Yet, buy-in at the clinical level is required for success in value-based care. Focused management of care pathways will ultimately provide better patient communication and monitoring to improve preventative care and minimize cost. It remains crucial for providers and healthcare systems alike to continue leveraging these advancements while confronting its challenges to accomplish CMS’s goal for accountable care relationships. Ultimately there are still very real rewards to be harvested from value-based care and risk-bearing models; we have seen examples of this in recent transactions. As margins for primary care continue to compress and new opportunities for top line revenue become scarcer, providers and investors who are able to correctly price risk and deliver greater value will be well positioned to thrive in the coming years.
How to Protect Your Business? Recommendations for Providers and Facilities based on Recent Enforcement Actions and Settlements
As criminal and civil enforcement efforts increase, proactive compliance efforts and stakeholder communication is key. Various federal agencies have recently increased their criminal and civil enforcement efforts and the sophistication of their enforcement activities. Industry insiders specifically noted an emphasis on areas such as telemedicine, controlled substances and the use of money received in connection with the COVID-19 pandemic. In response, organizations should frequently review their compliance programs to ensure they are comprehensive, consistent with best practices and any legal requirements, and well communicated to every stakeholder. Some best practices include: (i) establishing strong reporting channels that are emphatically encouraged by senior leadership (including those that are anonymous); (ii) creating processes for identifying and addressing potential issues quickly and effectively; (iii) building and broadly communicating specific plans and policies for exactly how to respond if a government inquiry is received or an investigation begins; and (iv) ensuring all federal forms, such as Medicare Provider Enrollment, Chain, and Ownership System forms (which have been the subject of heightened government scrutiny and are leading to increased recoupment efforts), are accurate and consistently updated with each applicable change. In addition to minimizing risk in these ways, it is important to strategically consider how to address, retain, or transfer the risks that remain. For example, while there have historically been very limited options for mitigating these kinds of risks, insurers are beginning to develop some risk-shifting products in this space. In this manner, organizations can minimize the risks that exist, address those that remain and proactively respond to those that materialize.